What is a Microsphere Used for?
Microspheres or micro-particles are complex drug formulations combining an Active Pharmaceutical Ingredient (API) with an FDA-approved polymer such as PLGA. Microsphere drugs enable the sustained release of APIs into patients over prolonged periods of time, varying from weeks up to several months.
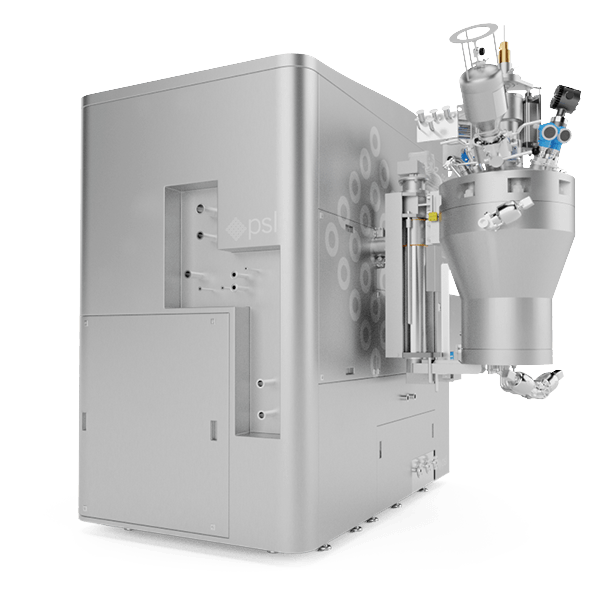
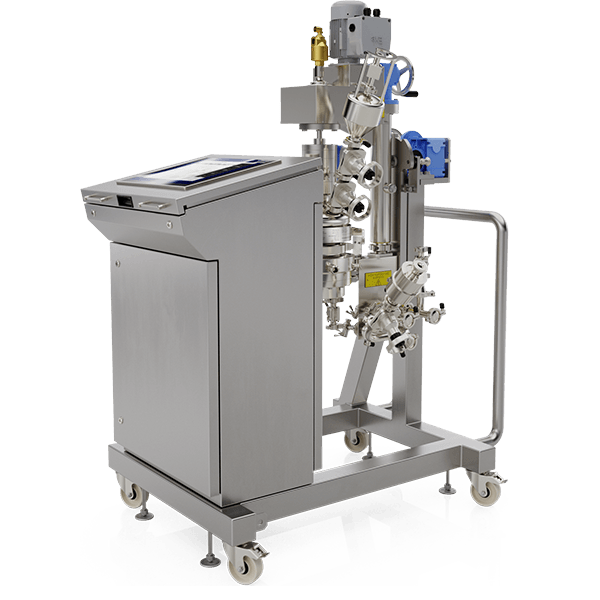
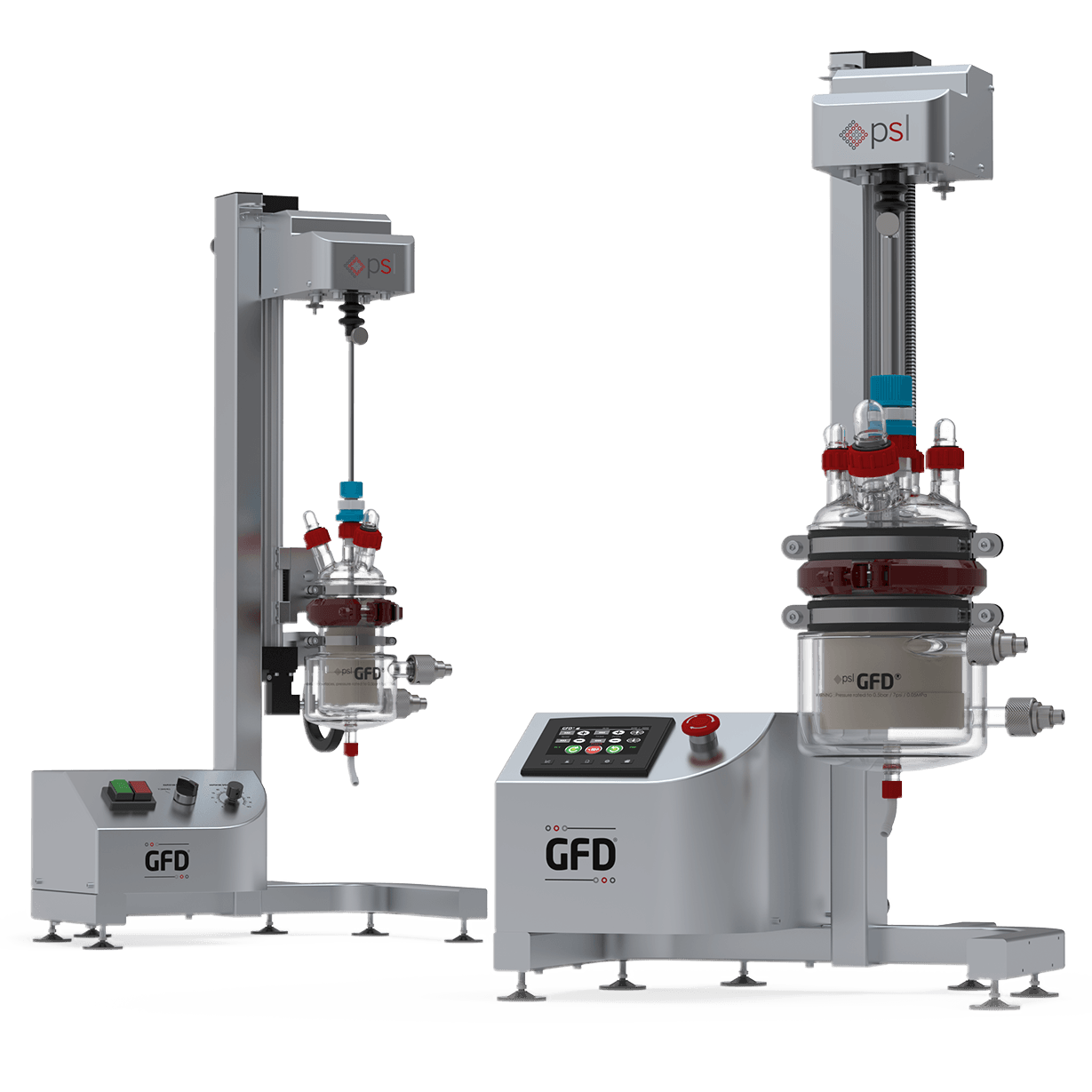
The MicroSphere Refiner (MSR™) is the flagship process technology of Powder Systems Ltd (PSL). It is disrupting the way microsphere drugs are traditionally developed and manufactured around the world, directly influencing drug delivery systems (DDS).
Watch our new video for more information.
Scalable Microsphere Production
MSR™ technology has been used worldwide and is proven at all levels of microsphere production, from early R&D activities up to clinical trials and commercial manufacturing. It has been utilised to successfully process well-known PLGA microsphere drugs incorporating various APIs such as Risperidone, Exenatide, Octreotide, Leuprolide, Naltrexone, etc.
Our industry references include global players from the Big Pharma as well as smaller drug innovators and generic drug manufacturers.
MSR™ Microsphere Technology
Innovative Drug Delivery

Despite their outstanding benefits for millions of patients around the world, microspheres are notoriously known to be difficult to process and develop for drug manufacturers.
Our unique MicroSphere Refiner (MSR™) technology was created to provide drug manufacturers with a reliable and innovative tool enabling them to overcome well-known issues in their microsphere scale-up development, such as filtration and drying inefficiencies, poor batch reproducibility and lack of sterility assurance.

A Legacy of Innovation
The MSR™ is a one-of-its-kind solution that has been developed over the last 25 years in collaboration with industry partners through a Quality-by-Design (QbD) approach, taking into account the microspheres characteristics and process behaviour.
PSL adopted a systematic approach to the development of the MSR™ technology by pre-defining industry-wide objectives for quality production of microspheres. A particular emphasis was put on process understanding and control through P.A.T. (Process Analytical Technology).

Worldwide Recognition

The unique features and benefits of the MSR™ approach have been recognised industry-wide enabling PSL to win global awards including the prestigious Innovation Award from ACHEMA 2015, the world forum and leading show for the process industries. Our technology also won the Queen’s Award for Innovation presented by Her Majesty Queen Elizabeth II in the UK.